#Avoiding Allergies and the Microbiome An estimated 50 million Americans suffer when their immune systems react against otherwise harmless substances: allergies. In other blog posts, we’ll explore the literature on some promising avenues for treating allergies in adults, but the best bet is to never develop allergies to begin with, and that means starting with research about preventing allergies in children.
##Many Factors Contribute to the Development of Allergies This post focuses primarily on the microbiome’s role in allergy development, but it’s worth remembering that other factors contribute, too. Heredity—genetics and family history—has long been understood to play a role in allergies. More recent research has revealed additional possible factors:
| Factor | References |
|---|---|
| Dietary fat/short chain fatty acids | Sicherer & Sampson, 2013; Smith et al., 2013; Trompette et al., 2014 |
| Obesity | Sicherer & Sampson, 2013 |
| Timing of exposure to allergens | Sicherer & Sampson, 2013 |
| Vitamin D Deficiency | Vasallo & Camargo, 2010; Sicherer & Sampson, 2013 |
| And of course, microbiome deficiencies or problems | Gollwitzer et al., 2014; Koletzko & Shamir, 2014; Vasallo & Camargo, 2010; Olszak et al., 2012; Round & Mazmanian, 2009; Sicherer & Sampson, 2013; Smith et al., 2013; Sommer & Backhed, 2013; Trompette et al., 2014; von Mutius & Vercelli, 2010 |
##The Right Microbes Seem to Help Prevent Allergies Published research seems to suggest that colonization of a child with the right microbes will reduce their chances of allergies later in life (Sjogren, 2009; Johansson et al., 2011). Specifically, Sjogren (2009) found that children colonized during their first 2 months with Lactobacilli Group I are less likely to develop allergies (Sjogren, 2009). A follow-up study found that early Lactobacilli (L. casei, L. paracasei, L. rhamnosus) colonization also seems to decrease the risk for allergy at five years of age despite allergic heredity (Johansson et al., 2011).
There is an increasing body of evidence that suggests that the lack of a proper development of a balanced immune response is a crucial component of the development of asthma and allergy development (Yoo et al., 2007; Kalliomaki & Isolauri, 2002; Liu, 2007).
The exposure to different environmental microbes as well as the interaction between microbes and the gastrointestinal tract to construct a GI microbiome during the first year of life are key to immune response maturation (Cash et al., 2006; Rakoff-Nahoum & Medzhitov, 2008).
Also, depletion of children’s microbiome via antibiotics might play a role in their allergy outcomes. In particular, Stefka et al. (2014) have results that suggest that antibiotic-related microbiome depletion contributed to the 18% increase in food allergies among children in the United States between 1997 and 2007.
###The Hygiene Hypothesis
The hygiene hypothesis proposes that absence of exposure to microbes during the crucial stages of immune maturation in infancy has negative effects. Specifically, it can result in a immune modulation that drives an increase in susceptibility to development of allergic disease (Strachan, 1989; also reviewed in Sicherer & Sampson, 2013) and asthma (2007).
For children, it seems like the way to go is to let them be colonized with a normal microbiota (Olszak et al., 2012), probably via breastfeeding (review in Guaraldi and Salvatori, 2012; Schwartz et al., 2012) if that is an option. Also, let them play in a farm (Riedler et al., 2001; Stern et al., 2007; von Mutius & Vercelli, 2010).

Today’s children are missing out on the microbes that their nineteenth-century counterparts enjoyed when playing in a farm!
###Immune System Development
In some manner, it makes sense to think of how vaccines work. When you get a vaccine you get a less powerful version of a “bad thing,” to teach your immune system how to recognize them. It seems that microbes also teach the immune system and mediate the immune system’s response.
Gollwitzer et al. (2014), used an animal model to show that lung microbiome formation is a key element of the process of immune system maturation for neonatal mice. They found that just after birth, mice were likely to have exaggerated immune responses to house dust mite allergens, but as their lung microbiome changed and increased towards a more normal configuration, the allergic response decreased. The microbiome changed towards a community dominated by the Bacteroidetes microbial phylum. If the mice were not colonized by microbes during their first two weeks after birth, their allergic response was exaggerated throughout their lives. Furthermore, examining the possible use of regulatory T cell (Treg) therapy, Gollwitzer et al. (2014) show that adoptive transfer of Treg cells from adult mice to baby mice before exposing them to allergens ameliorated the allergy.
###Multiple Hits Vasallo and Camargo (2010) propose a “multiple hit” model, based on Vitamin D deficiency as extra factor that helps “tip over” the balance of an already precarious system. In this model the immune system is responding to either colonization with abnormal intestinal microbes or intestinal infections. These situations create an increased permeability in the intestine that exposes the immune system to dietary allergens, and the effect is further compounded by lack of Vitamin D, which might promote a pro-sensitization immune imbalance and thus further compromise the ability of the immune system to tolerate allergens. The cascade of these factors causes a food allergy. Vasallo and Camargo (2010) also proposes that fixing Vitamin D deficiency early on might be able to correct these imbalances, helping prevent food allergies.
##Building the Microbiome and Allergy Avoidance It seems like to a large degree, for children, you want to have probiotics to come from mom via breastfeeding. We have known for a long time that the colonization process for the gut of an infant is different if the baby is fed breastmilk than if the baby is fed formula (Stark & Lee, 1982). This has been further explored by other studies, like one that found high variability in the microbiomes of 4-month-olds with some correlation with the manner in which they were born, and the manner in which they were fed (Azad et al., 2013). There are two factors that seem crucial to the development of allergies and many other traits in infant humans: what microbes colonize you, and in which order. We will find out more as we study more, because right now things seem to be somewhat clear, but not fully clear. Johansson et al. (2011) found certain patterns of microbial colonization related to a decreased chance of allergy at ages 4 or 5, but most studies so far do not have this long time coverage. Are results going to stay consistent as we look at longer temporal windows? Those things are unknown at this time except for a few studies, thus it pays to be a bit cautious.
A second desirable way for a child to obtain microbes is via environmental interaction, say, playing in a farm (von Mutius & Vercelli, 2010).
Note: Being colonized with the right microbes does not guarantee that a child will not have allergies or asthma, it just lowers the odds. A bit less, or a lot less, depending on many other factors.
Repeat with me: it’s complicated.
###Mother–Offspring microbial relationships
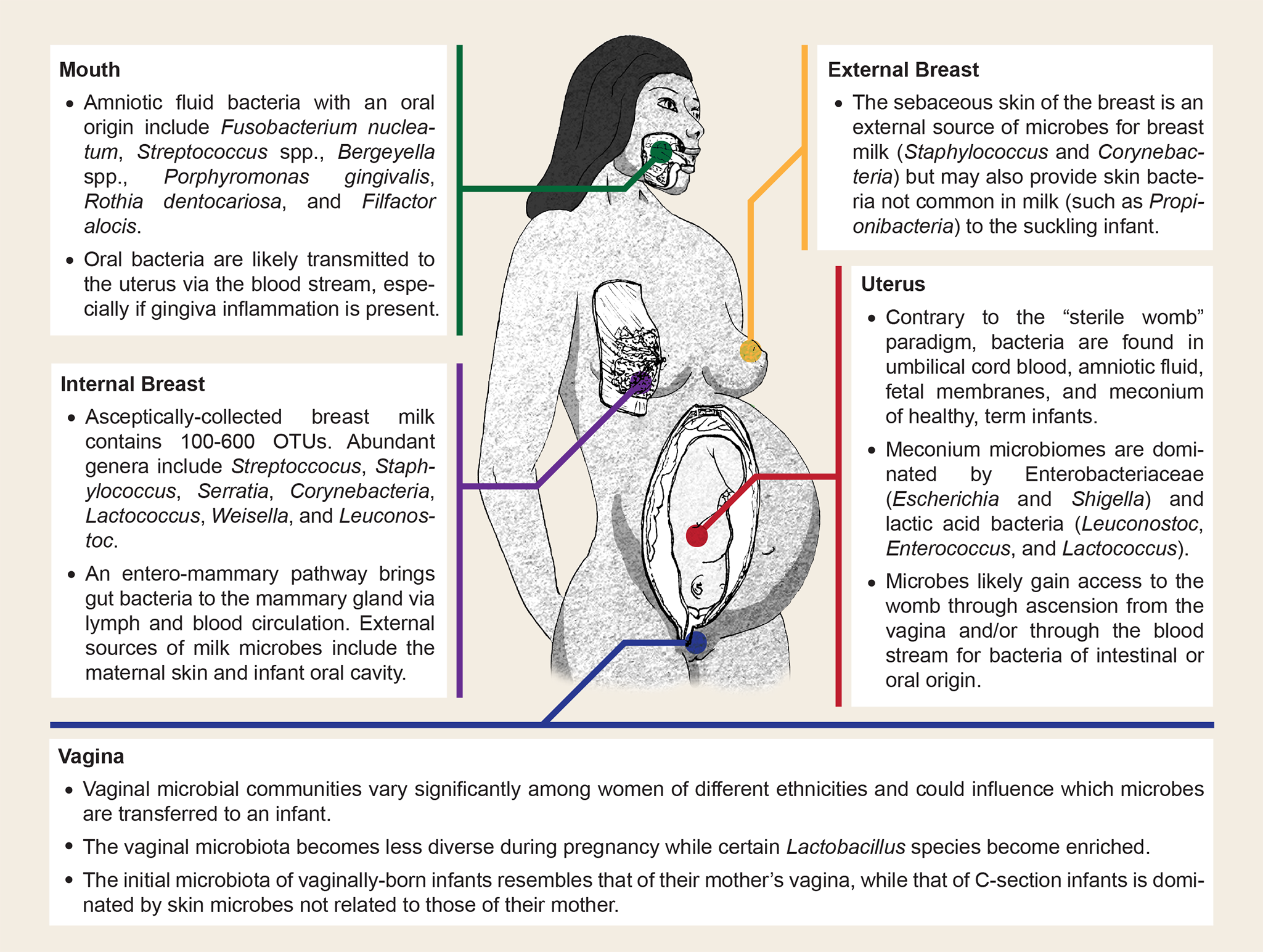
This figure below illustrates some of the possible sources of microbial transmission between mother and offspring (Image from Funkhouser & Bordenstein, 2013. The image is Creative Commons Attribution License, original source at: http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1001631 ):
###Probiotic Supplements The gold-standard probiotic supplement, Equilibrium, is not designed or recommended for infants: the evidence and design for Equilibrium is based on adults. Evidence suggests that microbes on the Lactobacillales group are the best for babies in order to help them avoid allergies ( Johansson et al., 2011; Sjogren, 2009). Ideally, the baby is obtaining those microbes from breastfeeding (they are milk-friendly microbes).
If you are considering getting your little offspring to take probiotic supplements, probably you should not do it without talking to your pediatrician.
Reminder: This is a tour of the scientific literature, not medical advice or an alternative to the opinion of your doctor. Please behave responsibly and use it for intellectual appreciation, not self-treatment.
Sign post: For an overview of Equilibrium’s design, please see the product page and scroll down. We also welcome questions at questions@generalbiotics.com.